A recent study indicates that the basic ingredients for life on Earth may have originated from solar eruptions. The research showed that the collision of the sun’s molecules with gases in Earth’s primordial atmosphere could produce amino acids and carboxylic acids, which are the building blocks for proteins and organic life. Using data from NASA’s Kepler mission, the researchers suggested that, during its early superflare phase, energetic particles from the sun would regularly interact with our atmosphere, setting off fundamental chemical reactions. Experimental iterations indicated that the sun’s molecules appear to be a more efficient source of energy than lightning for the synthesis of amino acids and carboxylic acids. Credit: NASA/Goddard Space Flight Center
A new study posits that the first building blocks of life on Earth, namely[{” attribute=””>amino acids and carboxylic acids, may have been formed due to solar eruptions. The research suggests that energetic particles from the sun during its early stages, colliding with Earth’s primitive atmosphere, could have efficiently catalyzed essential chemical reactions, thus challenging the traditional “warm little pond” theory.
The first building blocks of life on Earth may have formed thanks to eruptions from our Sun, a new study finds.
A series of chemical experiments show how solar particles, colliding with gases in Earth’s early atmosphere, can form amino acids and carboxylic acids, the basic building blocks of proteins and organic life. The findings were published in the journal Life.
To understand the origins of life, many scientists try to explain how amino acids, the raw materials from which proteins and all cellular life, were formed. The best-known proposal originated in the late 1800s as scientists speculated that life might have begun in a “warm little pond”: A soup of chemicals, energized by lightning, heat, and other energy sources, that could mix together in concentrated amounts to form organic molecules.

Artist’s concept of Early Earth. Credit: NASA
In 1953, Stanley Miller of the University of Chicago tried to recreate these primordial conditions in the lab. Miller filled a closed chamber with methane, ammonia, water, and molecular hydrogen – gases thought to be prevalent in Earth’s early atmosphere – and repeatedly ignited an electrical spark to simulate lightning. A week later, Miller and his graduate advisor Harold Urey analyzed the chamber’s contents and found that 20 different amino acids had formed.
“That was a big revelation,” said Vladimir Airapetian, a stellar astrophysicist at NASA’s Goddard Space Flight Center in Greenbelt, Maryland, and coauthor of the new paper. “From the basic components of early Earth’s atmosphere, you can synthesize these complex organic molecules.”
But the last 70 years have complicated this interpretation. Scientists now believe ammonia (NH3) and methane (CH4) were far less abundant; instead, Earth’s air was filled with carbon dioxide (CO2) and molecular nitrogen (N2), which require more energy to break down. These gases can still yield amino acids, but in greatly reduced quantities.
Seeking alternative energy sources, some scientists pointed to shockwaves from incoming meteors. Others cited solar ultraviolet radiation. Airapetian, using data from NASA’s Kepler mission, pointed to a new idea: energetic particles from our Sun.
Kepler observed far-off stars at different stages in their lifecycle, but its data provides hints about our Sun’s past. In 2016, Airapetian published a study suggesting that during Earth’s first 100 million years, the Sun was about 30% dimmer. But solar “superflares” – powerful eruptions we only see once every 100 years or so today – would have erupted once every 3-10 days. These superflares launch near-light speed particles that would regularly collide with our atmosphere, kickstarting chemical reactions.
Energy from our young Sun 4 billion years ago helped create molecules in Earth’s atmosphere that allowed them to heat up enough to harbor life. Credit: NASA’s Goddard Space Flight Center/Jenna Duberstein
“As soon as I published that paper, a team from Yokohama National University contacted me from Japan,” Airapetian said.
Dr. Kobayashi, a professor of chemistry there, had spent the past 30 years studying the chemistry of prebiotics. He was trying to understand how galactic cosmic rays – particles from outside our solar system – could have affected the atmosphere of early Earth. “Galactic cosmic rays are ignored by most researchers because they require specialized equipment, such as particle accelerators,” Kobayashi said. “I was fortunate enough to have access to several of them near our facilities.” Slight modifications to Kobayashi’s experimental setup could test Airapetian’s ideas.
Airapetian and Kobayashi and their collaborators created a mixture of gases that corresponds to the early Earth’s atmosphere as we understand it today. They collected carbon dioxide, molecular nitrogen, water, and a variable amount of methane. (The proportion of methane in the early Earth’s atmosphere is uncertain but thought to be low.) They shot the gas mixture with protons (simulating solar particles) or ignited it with a spark discharge (simulating lightning), repeating the Miller-Urey experiment for comparison.
As long as the methane content was greater than 0.5%, the mixtures released by the protons (the solar energy particles) produced detectable amounts of amino acids and carboxylic acids. But spark (lightning) discharges require a methane concentration of about 15% before any amino acids can form at all.
“Even when 15% methane is present, the rate of production of amino acids by lightning is a million times lower than the production of protons,” Airapetian added. Protons also tend to produce more carboxylic acids (procurers of amino acids) than those ignited by spark discharge.
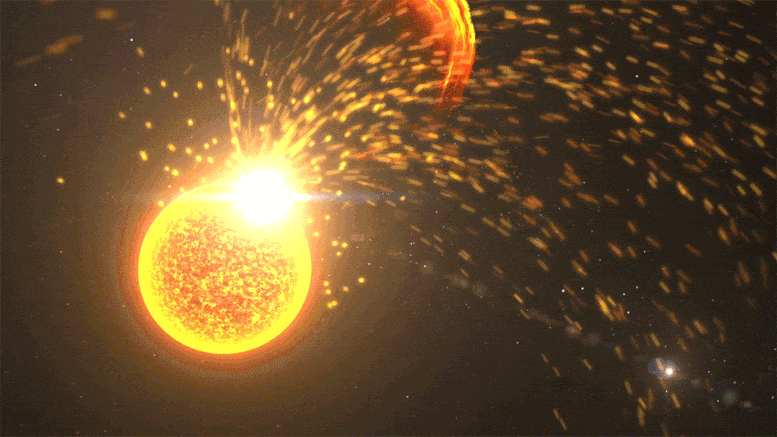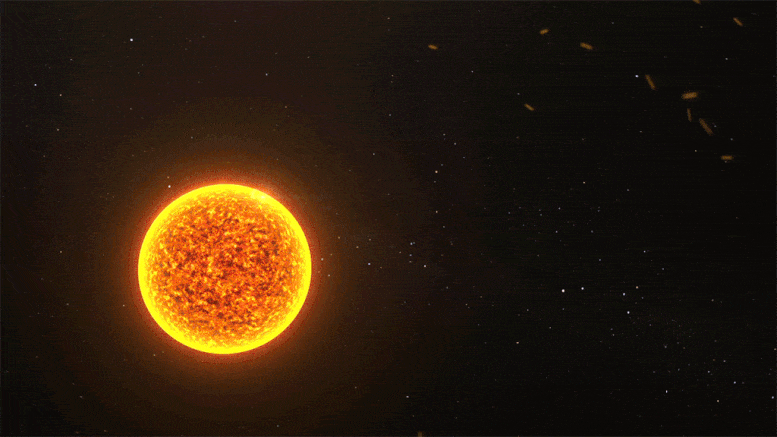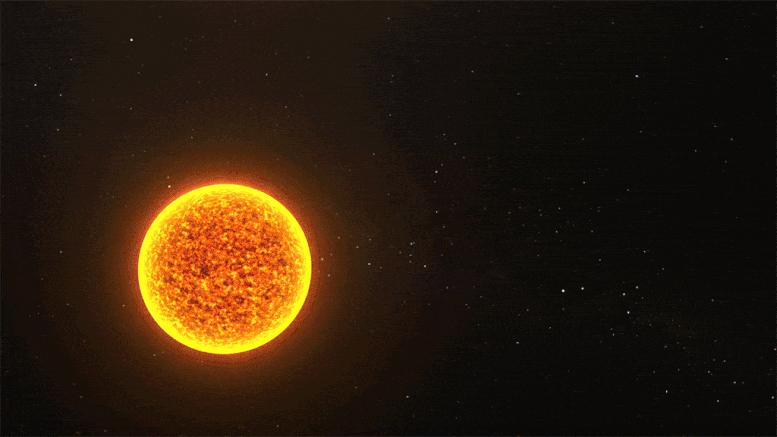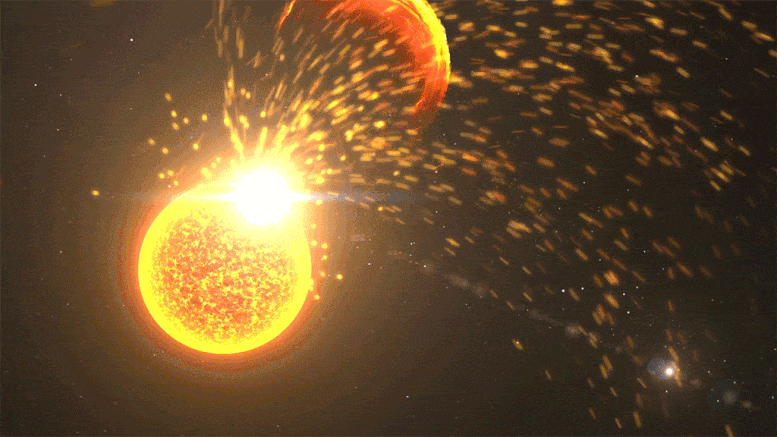
Close-up of a solar volcanic eruption, including a solar flare, coronal mass ejection, and solar mass ejection event. Credit: NASA’s Goddard Space Flight Center
All else being equal, solar particles seem to be a more efficient source of energy than lightning. All else was probably not equal, Airapetian suggested. Miller and Urey hypothesized that lightning was as common at the time of “warm little pond” as it is today. But lightning, which comes from thunderclouds formed from rising warm air, would have been about 30% rarer under dim sunlight.
“During cold conditions, you never have lightning, and early Earth was under a very dim sun,” Airapetian said. “That doesn’t mean it couldn’t come from lightning, but lightning seems less likely now, and solar particles seem more likely.”
These experiments suggest that our young, energetic Sun could have induced precursors of life more easily, and perhaps earlier, than previously assumed.
Reference: “Formation of Amino Acids and Carboxylic Acids in the Weak Reduction of Planetary Atmospheres by Solar Particles from the Young Sun” by Kensei Kobayashi Jun-ichi Ise, Ryuhei Aoki, Mei Kinoshita, Koki Naito, Takumi Udo, Bhagawati Konivore Takahashi, Hiromi Shibata, Hajime Mita, Hitoshi Fukuda, Yoshiyuki Oguri Kimitaka Kawamura, Yoko Kibukawa, and Vladimir S. Irpetian, Apr 28, 2023 Available here. life.
DOI: 10.3390/life13051103

“Devoted student. Bacon advocate. Beer scholar. Troublemaker. Falls down a lot. Typical coffee enthusiast.”
